Integrated Services
Kemwell is your one-stop for CDMO services for
mammalian cell culture products for both Drug
Substance and Drug Product
Step 1
KEMWELL TO DETERMINE & EXPLAIN
THE BEST PATHWAY FOR YOUR
PRODUCT DEVELOPMENT
Step 2
COLLABORATION THROUGH WELL
CHARTED PROJECT PLAN AND
DEFINED TIMELINES
Step 3
CELL LINE DEVELOPMENT IN
COLLABORATION WITH TRUSTED
PARTNERS
Step 4
PROCESS, ANALYTICAL AND
FORMULATION DEVELOPMENT
Step 5
SCALE-UP, cGMP CLINICAL TRIAL
MANUFACTURING & TESTING
(ANALYTICAL & GMP STABILITY)
Step 6
SCALE-UP, OPTIMIZATION & cGMP
COMMERCIAL MANUFACTURING
(DS AND FILL-FINISH)
Step 7
PRODUCT LIFE-CYCLE
MANAGEMENT

INTEGRATED DEVELOPMENT & MANUFACTURING
One-stop solution for mammalian cell-culture based protein therapeutics
- Monoclonal antibodies, bi-specific or multi-specific antibodies, and fusion proteins.
- End-to-end activities from cell line development to cGMP clinical and commercial manufacturing.
- Efficient transfer for process development, and analytical development activities.
- cGMP manufacturing of both Drug Substance & Drug Product at the same site
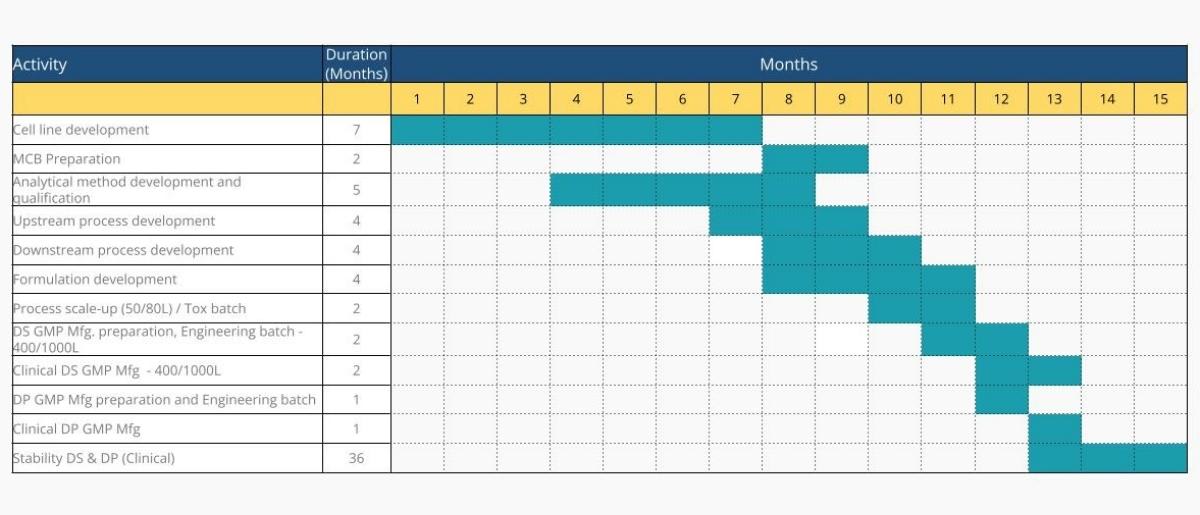
Cell Line Development to Clinical Phase 1
WHY KEMWELL
Being a trusted 100% biologics CDMO, all product related IPs are fully owned by our partners.
Kemwell uses advanced data protection tools and maintains the highest transparency with partners regarding the activities, reports, and documents. The team has implemented LIMS (for QC activities), CSV (for GMP activities) – in line with 21 CFR part 11, EU annexure 11 and GAMP 5- SAP (for batch release activities), EMPOWER server system (for tracking HPLC activities), live tracking systems (for all controlled areas) and created centralized lab servers for monitoring lab activities. The team is also on track to implement advanced e-QMS and e-DMS systems.